Fish Tank pH Levels (An easy guide for fishkeepers)
Whether you are a serious fishkeeper or just a gentle hobbyist, you should familiarize yourself with the subject of “Fish Tank pH Levels.”
Water pH (power of hydrogen) is measured on a scale of 0 (acid) – 14 (alkaline), with 7 being neutral. Optimum pH levels are between 6.5 and 8 for most freshwater fish. When fish tank pH levels become too high or low, fish can become stressed and even die.
If you find pH levels confusing, you are not alone. Understanding some of the terms used when discussing a fish tank’s pH level is essential, but it can also become overwhelming.

This article will explain aquarium pH levels in small, easy-to-digest chunks and in an easy-to-understand way, so read on to become an instant expert.
Fish Tank pH Levels (In Simple Terms)
The level of acidity in water is measured on the pH scale. The more acidic the water becomes, the lower the pH. The opposite of acid is alkaline. Water that is very alkaline typically contains minerals that absorb acids and make the pH of the water more stable.
If the water becomes highly acidic or alkaline, severe damage can occur to an aquarium’s entire ecosystem, and it can cause irreparable damage to the internal and external health of your fish, plants, and even the microbes in the aquarium.
All fish have a preferred pH which relates to their origin. Some areas of the world have higher levels of acid or alkaline in soil and rocks, which transfer to lakes and streams in which fish live. In most cases, achieving a balanced pH which is considered neutral, is the best way to suit a variety of fish that will slowly adapt.
Changing the pH level of aquarium water, in simple terms, requires you to add substances of the opposite chemical makeup. For example, to offset a high acidity level, you need to add small amounts of alkaline substances.
Although this explanation of aquarium pH is at the most basic level, it set a firm foundation for the following sections that will take you a tiny bit deeper. Don’t get put off by terms such as Buffers, Basic, KH, and GH. The information is provided at a level that is simple enough to keep your fish tank healthy but allows you to dig deeper if you want.
What Is pH In A Fish Tank?
pH in a fish tank is the acid or alkaline level in the water. The chemical makeup of the water in your aquarium is vital to keep your fish healthy so they can thrive. Matching your fish tank’s pH level to what your fish are used to in their natural habitat will create a more comfortable environment for them to live in.
Fluctuations in a fish tank’s pH level can be caused by decaying fish food, fish waste, and decaying plant matter. It is important to cycle your aquarium before adding fish, as fishless cycling will reduce fluctuations.
The pH level will fluctuate to some degree both in the wild and in freshwater aquariums. Most fish are used to small fluctuations, but more significant changes can be devastating to the health of your fish, your plants, and your aquarium’s overall biological health and ecosystem.
What does pH Stand For?
pH stands for “Pondus Hydrogenii,” which is Latin for the “weight of hydrogen” however, most people believe that pH stands for “Power of Hydrogen” or “Potential of Hydrogen,” and these terms have become more commonly used. Either way, the “H” is capitalized because hydrogen is an element on the periodic table written as “H.”
pH was first termed and described by Danish biochemist Soren Peter Lauritz Sorensen in 1909.
What Does pH Measure?
pH is a measure of how basic or acidic an aqueous solution is, based on the number and activity level of hydrogen ions.
Hydroxide ions (a combination of hydrogen and oxygen) also play a part in the variance of pH levels.
An acidic solution has more hydrogen ions, while basic solutions lack hydrogen ions. The more hydrogen ions in relation to hydroxide ions, the more acidic a solution becomes. You will notice I have used the word “basic” instead of “alkaline,” which I will explain later in this article.
The pH scale runs from 0-14, with 7 sitting in the middle, indicating neutral pH. Each 1-point movement on the scale in either direction is 10 times the previous measurement. It is important to understand this 10x factor because the further you get from a neutral pH, the more severe each 1-point change becomes.
pH Scale
I often hear the question, “What is pH in water?” Even with an explanation like the one above, they are no closer to understanding. The easiest way to understand pH is by using the pH scale.
An excellent visual scale like the one below will help. The below scale gives you an idea of the level of acidity or alkalinity of many well-known foods or fluids and where they sit on the pH scale, with pure distilled water sitting in the middle of the scale at neutral pH.
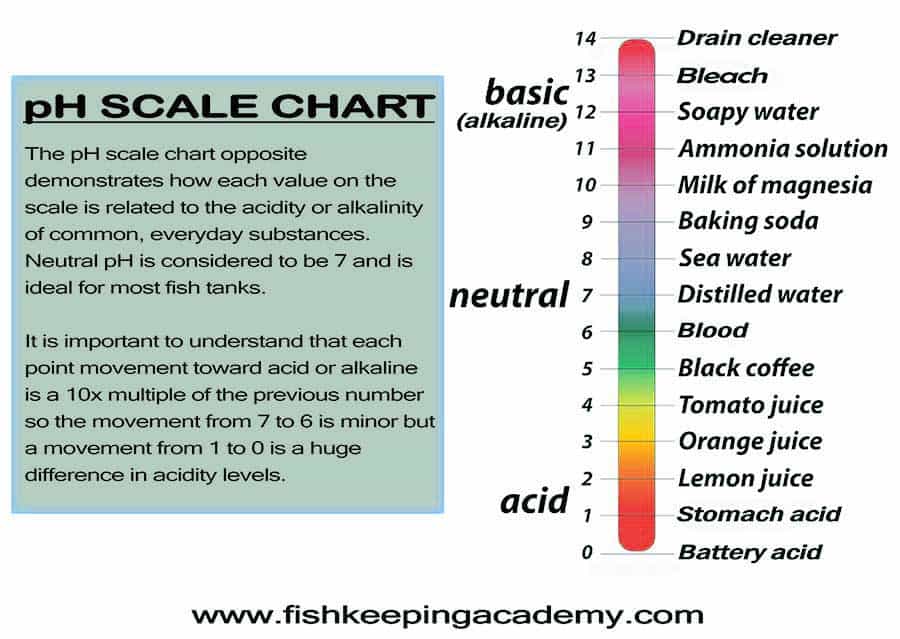
As a fishkeeper, a pH number scale like this is all you will need to understand pH levels at their simplest. Don’t get me wrong, there is far more to understanding how pH works, and I will cover some of it later in this article.
Your main concern is to ensure your fish tank water is not too acidic or alkaline (basic), which will harm your fish. By understanding the pH of your water, you will know whether you will need to raise or lower it.
Most pH test kits are easy to use and will return a result comparable to the pH scale.
Becoming familiar with pH values is an important step. You will need to know the ideal pH level for your fish species, why pH can rise or fall, and how to create a stable, balanced environment.
Why Is High pH Called Basic?
You will probably have noticed me referring to a high pH as “alkaline” and “basic” throughout this article. I will give a very brief explanation, but it’s not necessary to understand the difference between alkaline and basic as an aquarist.
Definition of Basic
In chemistry, a base is a water solution of any chemical compound that produces a solution with a hydrogen ion concentration lower than that of pure water. Sodium hydroxide and ammonia are two examples. Bases are the chemical opposites of acids. Bases reduce the hydrogen ion concentration in water, whereas acids increase them. Acids and bases neutralize each other when they combine.
Definition of Alkaline
In chemistry, the term alkali refers to salts (ionic compounds) containing alkali and alkaline earth metal elements that accept a hydrogen ion in solution. Alkaline bases are best known as bases that dissolve in water. Alkali metals react vigorously with water, producing hydroxides and releasing hydrogen. The reaction with air covers the surface of the solution with oxides. In nature, ionic compounds (salts) contain alkali metals but never in a pure state.
Source: Sciencing.com – Alkaline VS Basic
As far as this article, I will refer to a high pH as both alkaline and basic so that you will become familiar with both terms.
pH Buffers
If you have been reading about pH levels, you will have probably come across the term “pH buffers” or “pH buffering,” This is a subject worth understanding as it will help keep your aquarium pH stable.
What Is An Aquarium pH Buffer?
In short, buffering is the ability of water to resist changes in pH. Many chemical compounds can be used as aquarium pH buffers to protect against rising or falling pH levels.
Creating a stable pH is the perfect solution within any fish tank, and you can easily achieve it.
Again, I will not hit you with a ton of information about how chemical compounds interact, although this is generally how buffering works.
Water with a high mineral content is usually harder and more alkaline (higher pH), whereas water with a low mineral content is softer and more acidic (lower pH).
Water that is too alkaline would require the addition of acid to balance it out; however, the high level of minerals found in this type of water would buffer against the acid, causing very little change to the pH.
To remove this issue, you would first need to remove some of the mineral content before adding an acidic compound such as peat or a commercial pH decreaser.
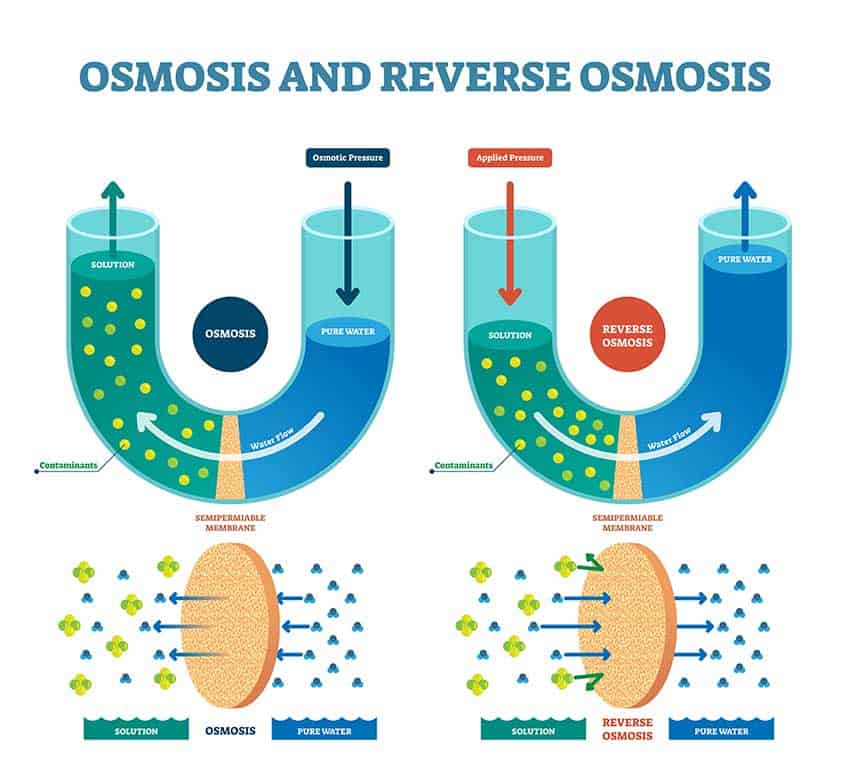
How do you remove minerals from water? One method is reverse osmosis, which filters minerals from the water using a special device. Another way of removing minerals is by changing and adding water back with lower mineral content. Distilled water can be used in your fish tank because it has no minerals and is pH neutral.
The opposite is true for raising pH. Instead of removing minerals, you need to add minerals to act as a natural buffer.
What Is Aquarium KH?
While talking about buffering and water hardness, I will briefly mention Carbonate Hardness (KH), also known as alkalinity or total alkalinity.
Carbonate hardness measures carbonates (CO3) and bicarbonates (HCO3) found dissolved in water. A high KH acts as a good buffer against acid, which will eat away at the KH before it affects your pH.
KH levels are not permanent and will gradually drop over time.
What Is The Difference Between KH And GH?
KH (Carbonate Hardness) and GH (General Hardness) are often confused as they both refer to water hardness. Despite the similarity, the parameters measured are entirely different.
I have already mentioned that KH measures carbonates and bicarbonates. GH refers to the amount of magnesium and calcium dissolved in water. GH and KH are usually pretty similar in nature; however, a water source such as tap water can have a high GH and low KH.
When discussing tap water, people are usually referring to its GH. The hardness of aquarium water is more often referred to as its KH.
Important Points So Far
1. Lower pH water is better for soft water fish.
2. Higher pH is better for hard water fish.
3. Higher pH levels are more mineral-rich with a higher buffering capacity and more stability.
4. Lower pH levels are less stable and prone to sudden changes.
5. A high KH (Carbonate Hardness) is a good buffer against acid, preventing a downswing in your water’s pH.
You can learn everything you need to know about aquarium alkalinity by reading: What Is Alkalinity In Aquariums.
How Does pH Affect Aquatic Life?
Whether you own coldwater fish, tropical fish, freshwater fish, or saltwater water fish, they are all affected by pH levels. The natural pH in freshwater aquariums will usually differ slightly from that in saltwater aquariums. The general pH of a freshwater aquarium will typically sit at 6.5 to 8.0 depending on the tap water used, while in a marine aquarium, the pH will be 7.9 to 8.5.
The pH level in a fish’s native environment results from natural minerals and other factors. If your aquarium’s pH is very different from what the fish are used to, it can harm your fish. Very acidic water can cause burns to a fish’s skin, eyes, and lungs. Basic (alkaline) water can also cause these areas to become irritated (although not classed as burns).
Stress is another problem that can result from a pH value that is too high or low. Stress can lower a fish’s immunity and open them up to many illnesses that can be more serious than the initial skin irritation.
A combination of skin irritation and a lowered immunity can often lead to fatalities in your fish, so keeping a normal pH level is far more important than you may realize.
How Often Should You Test pH In An Aquarium?
You should test the pH in your aquarium at least once every 4-6 weeks, and you may need to test in a newer tank more often until you find the natural baseline.
pH levels are generally a result of environmental impacts, so variations will usually be consistent. If your fish tank’s pH swings wildly up or down, this is unusual, and you need to identify the cause.
Generally speaking, pH is rarely a problem in most aquariums but can sometimes be affected by changes in water chemistry, such as ammonia spikes from a build-up of fish waste.
By tackling the cause of a pH change, it will often return to a normal level by itself.
To test aquarium water pH, you will either need to buy some relatively cheap test strips or an electronic tester that will be more expensive but provide more accurate readings and always be on hand.
When testing pH levels, keep a record for the first 4-6 months; you may notice any patterns forming, which can help you predict future fluctuations.
How To Test pH In A Fish Tank
The 3 most accurate ways of testing the pH in a fish tank are by using A pH testing solution, pH test strips, or a digital meter.
Solutions and test strips are the cheapest options in the short term, but they will need replacing from time to time. A digital meter is easier and quicker to use but will be more expensive in the short term and occasionally need calibrating.

pH Testing Solution Instructions
When using a solution, you need to:
- Take a measured water sample from the tank (usually into a test tube.)
- The instructions on the solution bottle will direct you to add several drops of solution to the sample.
- Mix the solution by gently shaking the test tube side to side and wait for the water to change color.
- Use the supplied color chart to find the matching color and pH value.
I have provided links below for the Saltwater and Freshwater solutions.
Although the Freshwater solution is often less expensive, both products are very similar and can be used for both water types. The main difference is the colors returned, which will be different, so you will need the correct color chart. I would suggest purchasing the correct kit for first use and keeping the color chart handy, so you can buy the cheaper kit in the future.
What I Use
Digital pH Meter Instructions
pH Test Strip Instructions
Test strips are much easier to use than a solution and are generally the cheapest option. To use a test strip, follow the below steps:
- Open the container and remove 1 test strip before closing the lid tightly to keep moisture from entering and ruining the other test strips.
- Fully submerge the test strip into the tank water for a few seconds and then remove it.
- Wait for 30 to 60 seconds until the test strip has changed color and settled.
- Compare the color against the included color chart pH section to find the correct pH value.
Most test strips will give readings for all of the important parameters within an aquarium, such as Ammonia, Nitrates, Nitrites, Carbonate Hardness (KH), General Hardness (GH), Chlorine, and pH, which is why you need to compare the correct area of the test strip to the correct area of the chart.
What I Use
Symptoms Of High pH In Fish Tank
There are several symptoms of high pH in a fish tank. A pH of 8.5 or higher is considered too high for most fish tanks and signals high alkalinity levels.
Some of the most common symptoms of a high pH are:
- High levels of toxic ammonia – Ammonia build-up can be very toxic to fish, good bacteria, and plant life. Ammonia should not be confused with ammonium, a symptom of a low pH. Ammonium is not considered toxic to fish.
- pH shock – pH shock typically occurs if pH changes suddenly. Symptoms may include general illness, loss of appetite, and sudden death.
- Fish seeming irritable – Twitchy fish jumping, scratching, shimmying, or acting out of character can be caused by high pH. The skin irritation caused by highly alkaline water can cause discomfort and, in the worst cases, skin infections.
- Algae growth – Algae will thrive in alkaline-rich environments. Algae will thrive most at a pH between 8.2 and 8.7. Green algae are most common, causing green cloudy water, covering the glass until it is difficult to see your fish.
- Fish breathing at the surface – High pH can have a negative effect on oxygen levels in the water. A slight rise in pH can cause oligotrophic water (rich in dissolved oxygen) to become eutrophic (lacking in dissolved oxygen). This can cause your fish to swim at the top of the tank, where oxygen first dissolves into the water and is likely to be more abundant.
Symptoms Of Low pH In Fish Tank
Several symptoms may be present if your fish tank has a low pH level (higher acidity). Symptoms of low pH in a fish tank include:
- Fish may become pale, lethargic, inactive, and tired – When pH levels drop, it can harm a fish’s metabolic rate. As a fish’s metabolic rate slows, cells can start to break down, and the body will literally eat itself (a state known as autophagy).
- Fish may stop eating as frequently – Again, caused when metabolism slows, a fish will not need as much food to survive.
- pH shock – This can occur when the water’s pH drops suddenly, making your fish generally appear unwell and, in some cases, cause sudden death, especially with more severe pH swings.
How To Lower pH In Aquarium
There are several ways to manually and naturally lower pH in an aquarium, so let’s look at some of the manual ways first.
Use Reverse Osmosis
Reverse osmosis is a way to filter out minerals from the water by using a filtration device specifically designed to filter out microparticles. Using reverse osmosis, you are basically purifying the water, turning hard water into softer water, and removing the minerals that buffer the water by neutralizing acids.
Once your water has been softened, it will be more susceptible to a drop in pH, thus helping both a natural drop occur or become more reactive when adding pH-lowering chemicals.
The reverse osmosis process usually creates a more neutral pH level without further intervention, although the equipment can be a little pricey.
Lower pH In Aquarium With Vinegar
Although it sounds strange, you can lower the pH in your tank with vinegar.
- The best method is to take a measured water sample from the tank and test it with a pH test kit. Once you have established the current pH of the water, you can add a small amount of white vinegar.
- As a rough guide, add approximately 1ml of vinegar per gallon of water. Mix the vinegar thoroughly and test the pH after 30-60 minutes.
- Once you have established the correct amount of vinegar to achieve the desired drop in pH, you can treat the rest of the aquarium water.
- Don’t add the vinegar directly to the tank as this can cause a sudden drop in pH and lead to pH shock; instead, remove and treat the water in stages, or just add the vinegar in stages (over 24 hours) until the total dose has been added.
Use API pH Down
A simpler method to lower pH is adding a commercially available chemical such as API pH Down, which will be available in most fish stores and is readily available to purchase online.
API pH Down is a nitrate-free, phosphate-free pH adjuster that will not cloud your tank or encourage algae growth.
Although pH Down does the same as white vinegar, you can be assured that the chemicals are completely harmless to your fish tank and will come with a comprehensive set of instructions.
What I Use
How To Lower pH In Aquarium Naturally
Using the methods above will lower your pH level, but the results are not long-term. This is fine if you understand the cause of the fluctuation and can remove the cause. Still, if you are experiencing a permanently high pH, a more natural remedy may be a better solution and give more long-term results.
Peat Moss To Lower pH In Aquarium
Peat moss is a great way to lower the pH of your aquarium, as it is naturally acidic and can be added beneath the substrate, out of sight.
The acidity of the peat moss will slowly dissolve into the water and eat away at any buffering chemicals until the pH begins to normalize and gradually soften your aquarium water.
Catappa Leaves (Indian Almond Leaves)
Catappa leaves (also known as Indian almond leaves) come from a tropical tree found in regions of Africa, Asia, and Australia.
I learned about the Catappa leaf when treating betta fish ailments, as it has many therapeutic and medicinal benefits to fish.
These leaves are rich in tannins which will turn your tank water a shade of brown, but they are wonderful for regulating the pH levels in your aquarium.
Driftwood
Driftwood is another natural method of lowering pH because, like Catappa leaves, it also contains pH-lowering tannins.
Using natural driftwood with the highest tannins level is essential without added chemicals.
Do Live Plants Lower The pH Of Aquarium Water?
Live plants don’t lower the pH of an aquarium; they increase the pH level. During the daytime, live plants absorb carbon dioxide to produce oxygen. Carbon dioxide helps lower the pH of aquarium water, while oxygen has no effect on water pH.
If you have an overgrowth of plants, try removing some of them. Just a slight reduction of plants will help lower the oxygen content in the water, making room for some acidic carbon dioxide to lower the pH level.
Finding the right balance will make your tank look good, are essential to the diet of many fish species, and provide a good balance between oxygen and carbon dioxide.
Live plants are great for aquariums and, with regular care and cleaning, will help with many natural functions within the tank, leading to a healthy, oxygen-rich, bioactive environment.
Add Carbon Dioxide
Although you can add carbon dioxide manually to a fish tank, there are more natural ways, such as adding organic matter, which will release carbon dioxide as it decays.
Allowing organic matter to decay is a safe process that will happen over time, having a more stable and long-term effect.
When in water, carbon dioxide will act as an acid, which will help normalize the level of alkaline over time.
It is possible to add carbon dioxide manually; however, the effect would be more sudden, potentially harming your fish.
How To Raise pH In Aquarium
Understanding how to raise your aquarium water pH is just as important as lowering it, so let’s consider raising it manually first.
Water Changes
Regular water changes are the easiest way to keep your pH level stable. Most tap water will contain minerals that will help buffer against pH changes.
You should always check the pH of the tap water in your area before adding it to your tank. It is important to know the chemical makeup of the tap water you are using to treat it correctly and match it to the type of fish you have.
Aerating The Water
Adding dissolved oxygen to your aquarium through aeration does not directly impact pH; instead, it will reduce the level of acidic carbon dioxide, enabling the pH level to rise.
Aeration can be achieved by surface water disturbance, such as a good filter flow rate at the top of the tank or just stirring/splashing the surface water.
How To Raise pH In Fish Tank With Baking Soda
Baking soda is very alkaline and can raise the pH quite quickly. With any method that causes a quick change in the pH of your aquarium water, you will need to either do it in stages or remove your fish to a separate holding tank until completed.
The best method to follow when using baking soda to raise pH is:
- Take a small water sample (this can be 1-10 gallons, dependent on your tank size).
- Test the water with pH test strips before adding any baking soda. This way, you can gauge how quickly it changes.
- Add the baking soda to the water sample. 1 teaspoon of baking soda per 5 gallons will usually raise the pH level by around 1 point if it isn’t too far from neutral.
- Stir the baking soda into the water allowing it to dissolve, and sit for 30 minutes before re-testing. Take note of how much the pH rises.
- If you are going to remove your fish from the main tank, you can add some of the sample water into a holding tank, mixing some of the main tank water with it. If the pH rises too quickly, your fish may suffer from pH shock, so you want to avoid this.
- Treat the remaining main tank water with baking soda using the same ratios on the sample water.
- Once your main tank has reached the correct pH, you will need to gradually mix small quantities into the holding tank (for 12-24 hours) so that your fish get used to the change.
- Add your fish back to the main tank.
If you only require small adjustments to your aquarium’s pH, it may be better to use some of the more natural methods, which will happen over time and be a more permanent solution.
API pH Up
API pH Up is the opposite of the API pH Down formula above, slowly neutralizing the acid in your fish tank without harmful phosphates or other chemicals. It doesn’t promote algae growth like other methods.
What I Use
How To Raise pH In Aquarium Naturally
Natural changes are often best because they are more permanent and gradually affect water chemistry. Water remains relatively stable in a fish’s natural habitat because of many environmental factors, such as water’s mineral content and oxygen content.
Here are a few ways to naturally raise the pH in your aquarium.
Add Live Plants
Plants are the perfect natural source of oxygen and have many other benefits in creating a bioactive environment. As mentioned above, carbon dioxide is acidic in water, and aerating your fish tank will combat this problem. Live plants will naturally remove carbon dioxide from the water while adding oxygen.
Add Crushed Coral Or Dolomite Gravel
Adding minerals such as those in crushed coral or dolomite gravel will create a buffer that protects against acidity, allowing the pH to rise.
You can add these substances directly to the substrate in your tank for a slow increase in mineral levels, or you can put them in a netted media bag (Find The Cheapest Price) and place them within the filter element. This way, the minerals will be distributed more quickly through your aquarium.
Using crushed coral or dolomite is a long-term buffer that will help create a more stable pH environment.
Use Limestone Or Coral Rock For Decoration
Limestone or coral rocks will act the same way as the crushed coral or dolomite mentioned above. They are both mineral-rich and will create a buffer to absorb excess acid from the water.
You can purchase ornaments and features made explicitly from limestone or coral rock, which you can use to decorate your tank and act as a permanent pH buffer.
Remove Driftwood
Many people use driftwood as decoration in their tanks without realizing that the natural tannins released are acidic, lowering the water’s pH value. If you are struggling with a low pH, simply removing some or all driftwood from the aquarium should improve things.
Changes to pH will be gradual when adding or removing mineral-rich objects because minerals take time to dissolve into and away from the water. Natural buffers will take even longer to take effect in larger tanks, but creating a stable, balanced environment is worth the wait.
Conclusion
Water pH can be a very confusing topic, especially when looking at it from a scientific point of view. There are many confusing terms, such as General Hardness (GH) and Carbonate Hardness (KH), or Alkaline and Basic, which both refer to Alkaline but in different ways.
I am by no means an expert on water pH, and I have only ever researched as far as is necessary, so don’t be disillusioned if you have reached this far and are still confused.
As far as fishkeeping is concerned, you only need to understand a few things about pH. How to measure your pH, why it can increase or decrease, and learn some methods to raise or lower your pH quickly, naturally, and over time.
Many people believe that aquarium fish need to live in a specific water type and bang their heads against a wall trying to achieve this perfect value. Yes, some fish are better suited to particular water parameters, but most fish will adapt if the pH is neutral and stable.
You should initially test your fish tank’s pH regularly and not allow your pH values to increase or decrease by more than 1-1.5 points over 24 hours. Most freshwater fish tanks will do just fine within the range of 6.5 and 8. Slightly more alkaline water is safer and more stable than a lower acidic level.
Cycle a new aquarium properly before adding fish, and try not to make any sudden changes to the water chemistry. All water parameter changes should be slow and steady so they don’t impact your fish’s health.
I hope this article has been helpful in explaining the important aspects of pH levels and their role. You have done very well if you managed to reach the end of the article without falling asleep.
Try not to become obsessive about pH levels; treat it as just one piece of the puzzle regarding your fish tank’s water parameters. Many, far more important things can throw your water parameters into disarray, and most of them can be avoided by having a regular schedule for cleaning and basic fish tank maintenance.











